1.1 General Properties of Group 16 Elements and their Compounds
1. O, S, Se, Te and Po constitute group 16 of the periodic table. These are collectively known as chalcogens (ore forming). Their general valence shell electronic configuration is $ns^2np^4$.2. Atomic and ionic radii increases down the group while ionisation enthalpy and electronegativity decreases with increase in atomic number. Because of compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur. However, from ~ sulphur onwards the value again becomes less negative upto polonium, Po.
3. The elements of group 16 have lower ionisation enthalpy values compared to those of : group 15 in the corresponding periods, this is due to extra stable half-filled p-orbitals in group 15 elements.
4. The elements of group 16 show a number of oxidation states. Oxygen shows -2 oxidation State except in case of $OF_2(+2), O_2F_2, (+1) \ and \ H_2O_2(-1)$. Other elements of this group exhibit +2, +4 and +6 oxidation states but +4 and +6 are common. The stability of +6 oxidation state decreases and +4 oxidation state increases down the group due to inert pair effect.
5. All these elements form $H_2M$ (where, M = O, S, Se, Te, Po) type hydrides. Acidic character and reducing character of hydrides increases from H,S to H,Te. Bond dissociation enthalpy, thermal stability and HMH angle decreases down the group. Boiling points increase from $H_2S$ to $H_2Te$. $H_2O$ has extraordinarily high boiling point due to intermolecular H-bonding. $H_2O$ does not possess reducing property.
6. These elements form $MO_2$, and $MO_3$, (where, M = S, Se, Te, Po) type oxides. $SO_2$, is reducing while $TeO_2$, is an oxidising agent. Both types of oxides are acidic in nature.
7. These elements form $MX_2, MX_4 \ and \ MX_6$ type halides (X = F, Cl, Br and I). All hexafluorides are gaseous and octahedral in shape. $SF_6$ is exceptionally stable for steric reasons. $MX_4 \ (SF_4,SeF_4 \ etc.)$ type halides have $sp^3d$-hybridisation and have trigonal bipyramidal structure in which one of the equatorial positions is occupied by a lone pair of electrons (see-saw geometry).

8. Dihalides are $sp^3$-hybridised and have
tetrahedral shape. Examples are $S_2F_20, S_2Cl_2,S_2Br_2, Se_2Cl_2$ and $Se_2Br_2$. These
dimeric halides undergo disproportionation
as given below:
$2Se_2Cl_2 \longrightarrow SeCl_4 +3Se$
9. Oxygen is the first member of Group 16 with electronic configuration $1s^22s^22p^4$. $O_2$ and $O_3$ are its most important compounds.
(i) Dioxygen ($O_2$) in vapour state is paramagnetic in nature.
$2Se_2Cl_2 \longrightarrow SeCl_4 +3Se$
9. Oxygen is the first member of Group 16 with electronic configuration $1s^22s^22p^4$. $O_2$ and $O_3$ are its most important compounds.
(i) Dioxygen ($O_2$) in vapour state is paramagnetic in nature.
(ii) Ozone is an allotropic form of oxygen.
Due to the ease with which it liberate
atoms of nascent oxygen, it acts as a
powerful oxidising agent.
(iii) The two oxygen-oxygen bond lengths in $O_3$ molecule is identical due to resonance and the molecule is angular.

(i) Both allotropes of S, i.e. rhombic and
monoclinic sulphur have S, molecules.
The $S_8$ ring in both the forms is
puckered and has a crown shape. In
cyclo $S_6$, the ring adopts the chair form.
(ii) $SO_2$ is produced as a by-product of the roasting of sulphide ores. It behaves as a reducing agent (when moist). The two S—O bonds in $SO_2$ molecule are equal due to two resonating structures. $SO_2$ molecule is angular.
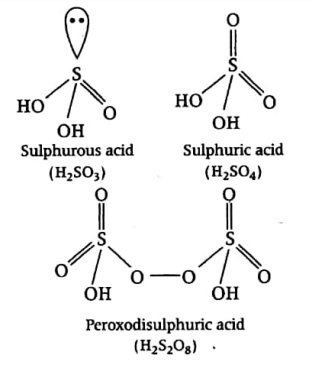
(iii) Structures of some important oxoacids of sulphur are given below:
(iv) Sulphuric acid is manufactured by contact process. It forms two series of salts; normal sulphates and acid sulphates. The larger value of $K_a$ indicates that $H_2SO_4$ is largely dissociated into $H^+$ and $HSO^-_4$ ions. Greater the value of $K_a$, the stronger is the acid.
Contact process involves following steps:
(iii) The two oxygen-oxygen bond lengths in $O_3$ molecule is identical due to resonance and the molecule is angular.

10. The second member of group 16 is sulphur.
Sulphur $(S_2)$ in vapour state is also
paramagnetic in nature. Its main oxides are $SO_2$ and $SO_3$ . Sulphur also form many
important oxo-acids. (e.g. $H_2SO_4$).
(ii) $SO_2$ is produced as a by-product of the roasting of sulphide ores. It behaves as a reducing agent (when moist). The two S—O bonds in $SO_2$ molecule are equal due to two resonating structures. $SO_2$ molecule is angular.
(iii) Structures of some important oxoacids of sulphur are given below:
(iv) Sulphuric acid is manufactured by contact process. It forms two series of salts; normal sulphates and acid sulphates. The larger value of $K_a$ indicates that $H_2SO_4$ is largely dissociated into $H^+$ and $HSO^-_4$ ions. Greater the value of $K_a$, the stronger is the acid.
Contact process involves following steps:





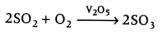






Thank you for consolidated notes for group 16 elements. 😇
ReplyDelete